ALPHA5BETA1 integrin
Despite various studies conducted on skin affected by vitiligo, the exact pathogenesis of this dermatosis has yet to be defined and many pathogenetic mechanisms have been put forward over the years; in particular, autoimmunity, cytotoxic metabolites and genetic or neural components. No convincing model that describes the interactions between all these factors has ever been identified. With our studies, we have confirmed that the most important predisposing factor to the development of vitiligo is a deficit of adhesion of melanocytes and that mechanical traumatism or various chemical stress factors may be the main precipitating events. Vitiligo is therefore a disease caused by a chronic detachment of melanocytes, and melanocytorrhagia is the transepidermal loss of these cells.
The interactions between melanocytes and the basal membrane are mediated by different molecules and in particular by alpha5beta1 integrins. The loss of adhesiveness of alpha5beta1 integrins caused by traumatism or by chemical stress factors is the first pathogenetic step that leads to vitiligo. However, that first step is never seen in normal skin, even though traumatism and chemical stressors may still be present. There must therefore be a sort of priming factor that is activated to facilitate the development of vitiligo patches. This factor has been identified as the MIA protein.
The active detachment of melanocytes
The active detachment of melanocytes and alpha5beta1 integrins are also involved in the context of malignant melanoma. Malignant melanoma is the most serious skin cancer and its metastatic form is associated with the worst prognosis. Metastatic dissemination appears to be mediated by an active detachment of the tumour cells and this detachment is caused by a small protein secreted directly by the melanoma cells, known as “Melanoma Inhibitory Activity” (MIA). It has been demonstrated that MIA interacts with the alpha5beta1 integrins by binding to those proteins on the cell surface and modulating their activity, thereby causing the detachment of the cells from the extracellular matrix. It became clear therefore that the interactions between a protein produced by those malignant melanocytes (MIA) and a melanocyte adhesion molecule (alpha5beta1 integrin) are responsible for the active detachment of neoplastic melanocytes in the context of malignant melanoma.
Our studies were therefore aimed at investigating the expression of the MIA protein also in the skin of patients suffering from vitiligo and its relationship with alpha5beta1 integrins, with a view to clarifying the possible pathogenesis of this disease.
The study on tissue samples
Ten samples of skin affected by vitiligo were included in our laboratory studies. These samples were taken from the edge of the vitiligo patches and included both normal pigmented and depigmented skin. All tissue samples were collected for diagnostic purposes. Five samples of macroscopically normal pigmented skin were selected as controls. At the time of collection, all patients did not have any familiarity for melanoma and underwent clinical screening to certify the complete absence of that skin neoplasm; furthermore, those patients were not undergoing any treatment for vitiligo.
Nine out of ten biopsy samples tested positive for the presence of MIA. The only MIA-negative sample was taken from a patient actually suffering from segmental vitiligo (SV) while all MIA-positive samples were taken from patients suffering from non-segmental vitiligo (NSV). All control samples were negative for MIA expression. This data shows that 100% of the samples suffering from non-segmental vitiligo were positive for MIA expression.
The exact pathogenesis of vitiligo is still in question and none of the models offered thus far is able to explain adequately the biological behaviour of the disease. The autoimmune hypothesis is currently the most agreed theory; however, it has never been able to explain the lack of clinical signs compatible with an inflammatory process (characteristic of every autoimmune disease) in vitiligo lesions or the greater presence of vitiligo patches in well-defined anatomical sites (such as the face and the acral regions): every autoimmune disease, by definition, should be distributed randomly over the affected organ. Therefore, in line with a non-autoimmune hypothesis for the pathogenesis of vitiligo, we focused on the role of MIA and integrins. The presence of MIA in non-tumour melanocytes was completely unexpected, as normal melanocytes do not express this molecule.
We therefore indicated a new pathogenetic model for non-segmental vitiligo.
The role of MIA
The normal anchoring of melanocytes to the basal membrane mediated by alpha5beta1 integrins is disturbed by the MIA protein. That first pathogenetic step is necessary and essential for the development of vitiligo (the “priming factor”) and as a consequence the secondary pathogenetic stimuli such as physical traumatism, oxidative stress or autoantibodies may lead to the loss of melanocytes by skin exfoliation (together with the “torrent” of keratinocytes which flow from the bottom upwards in the epidermis and which then drag away any melanocytes that are not anchored down). This model effectively explains the lack of clinical signs of inflammation in vitiligo patches and the greater presence of such patches in well-defined anatomical sites. According to our hypothesis, the melanocytes are therefore not destroyed by the immune system, but simply leave “in silence”. The melanocytes detach from the basal membrane towards the stratum corneum and exfoliate together with the surrounding keratinocytes. This process is entirely compatible with the absence of an inflammatory response.
In conclusion, these studies demonstrate that MIA protein is present in skin affected by non-segmental vitiligo and that it may cause the detachment of melanocytes, leading to the formation of achromic patches in response to various stimuli without causing any inflammatory process in the skin tissue. As already noted in malignant melanoma, also in vitiligo the target of MIA is represented by alpha5beta1 integrins, thus determining the breakage and/or weakening of the connections between melanocytes and the basal membrane. All these observations help to piece together the intricate puzzle of vitiligo and open the door to new therapeutic paths, thanks to the development of specific targeted therapies to inhibit the action of MIA for the treatment of non-segmental vitiligo.
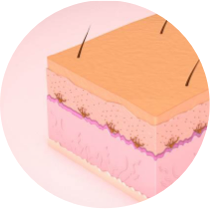
1. The melanocyte secretes the MIA protein and triggers the development of white patches
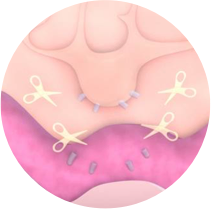
2. The MIA protein breaks the bond of the melanocytes with the basal layer

3. The melanocyte detaches and causes the formation of depigmented patches
